Introduction
Glaucoma is one of the most common ocular diseases leading to the progressive loss of visual function (1). It is characterized by neurodegenerative phenomena including chronic axonal damages and retinal ganglion cells (RGCs) loss, that lead to severe impairments of the signal pathway towards the visual cortex (2). To date, the multifactorial complexity of glaucoma is still far to be elucidated. The intraocular pressure (IOP) elevation is considered as the most recurrent risk factors driving the onset and the progression of glaucoma, acting as a pivotal pathophysiological factor for the RGCs neurodegeneration and optic nerve damage (3). However, it is increasingly clear that considering the IOP elevation as an overall pathogenic mechanism for RGCs degeneration in every glaucoma subgroup is limiting. In effect, ocular hypertension could not correlate with RGCs degeneration or even not occur as the case of normotensive glaucoma (4). This controversial scenario is also reflected in experimental models used in basic and pharmacological research on glaucoma, which are necessarily based on currently known disease triggers and risk factors. Indeed, several experimental models of glaucoma in vivo are mainly based on the induced elevation of IOP following surgical procedures such as intracameral injections of viscous solutions, intracameral injections of nanoparticles or episcleral vein occlusion in rodents (5). These models are intensively used for the study of the structural and functional outcomes deriving from ocular hypertension, which in this model is well controlled in its onset and progression. However, the lack of the pathophysiological dynamicity characterizing glaucoma restricts these models to the study of those degenerative phenomena deriving exclusively from the IOP elevation. A higher dynamicity in glaucoma onset and progression can be obtained by using inherited models of glaucoma. Among them, the DBA/2J mouse model is currently the best studied and used for modeling the progressive glaucoma pathophysiology since it displays an age-dependent phenotype similar to that in humans (6). However, the stressors driving the glaucomatous degeneration in this model cannot be strictly controlled, thus making this model highly variable. Therefore, the choice of the animal model should be made based on the precise aim of the study and the glaucoma phenotype/molecular mechanisms being assessed.
The present article summarizes experimental procedures, analytical methodologies and major outcomes descriptions related with two experimental models of glaucoma belonging to the classes of induced IOP-elevation and genetic models.
Experimental models of induced IOP-elevation
The regulation of IOP is mainly attributed to structural and functional mechanisms regulating the aqueous humor outflow. Relevant tissues of the anterior segment that are anatomically involved in IOP control include the ciliary muscles, trabecular meshwork, Schlemm’s canal, collector channels, and aqueous veins (7). Therefore, experimental alterations of the IOP can be obtained by surgical interventions impairing tissues functions in the regulation of the aqueous humor outflow. The surgical manipulation of this ocular region can be subdivided according to the mechanism of IOP elevation into post- and pre-trabecular (8). Post-trabecular models involve various treatments of the episcleral veins, such as laser coagulation, cauterization, or hypertonic saline injection. Post-trabecular models achieve a rapid but short-lasting elevation of IOP, although they have many disadvantages in rodents due to the small size of the eye (9). Pre-trabecular models are represented by intracameral injections of different substances hindering the aqueous humor outflow internally. The latter model is the most recurrent in the study of IOP-elevation in basic and pharmacological research since it allows a reproducible IOP-elevation without excessive manipulations of the eye. Among the substances used for the pre-trabecular model, several combinations and types of microbeads (polystyrene, magnetic) or viscous solutions are commonly used. Noteworthy, the use of microbeads has been highlighted as effective in inducing a significant increase in IOP, although their composition in could potentially exert toxic effects at the levels of anterior eye tissues. Therefore, the use of inert viscous solutions made of biocompatible polymers such as hyaluronic acid or methyl cellulose (MCE) would be preferred for their ability to induce an IOP-increment with a low rate of toxicity phenomena.
MCE model of induced IOP elevation
MCE is the methyl ether of cellulose, produced by reacting methyl chloride and alkali cellulose. It contains 27.58–31.5% of methoxy groups. MCE is reported in the USP/NF, Ph. Eur., JP, and FCC for its use in oral solid pharmaceutical formulations as a binder, coating agent, and as a controlled release matrix. MCE is available in a wide range of molecular weights, but almost exclusively the low molecular weight grade with nominal viscosity of 15 cps at 2% w/v concentration is used as a tablet binder (10). Contrariwise, upon proper preparation, the high molecular weight MCE in aqueous solution undergoes gelation with a mean viscosity reaching 4,000–5,000 cps (11). The latter is suitable for pre-trabecular models following intracameral injections aiming at altering the aqueous blood flow.
Procedure
Preparation of a 2% w/v MCE solution
-
Add 20 g of MCE powder (MW: 88.000 kDa) to 1 liter of distilled water and bring to a boil for 5–10 min using a heating magnetic stirrer.
-
Sterilize the solution immediately after the heating step by autoclaving for 16 min at 121 ℃ and 15 psi steam pressure.
-
Let the solution cool overnight at room temperature to obtain complete dispersion. The solution should be cloudy but uniform.
-
The solution can be stored at room temperature up to one year under sterile conditions. The solution cannot undergo further autoclaving sterilizations once prepared since it could lose its homogeneity.
Intracameral injection of the MCE solution in mice
-
The injection of MCE can be performed in C57BL6/J adult mice (8–12 weeks of age). The choice of the mouse strain could differ for experimental needs, but the age of the mice should be maintained in the range indicated in order to avoid effects of development or aging.
-
Perform the anesthesia by mean of an intraperitoneal injection of Avertin (1.2% tribromoethanol and 2.4% amylene hydrate in distilled water, 0.02 mL/g body weight; Sigma-Aldrich, St. Louis, MO, USA) and monitor the animal until deep sleep is reached in a temperature- and air-controlled environment.
-
Load a Hamilton syringe equipped with a 36-gauge beveled needle with the 2% w/v MCE solution. Make sure to avoid bubble formation within the loaded volume.
-
Insert the needle in the peripheral region of the cornea maintaining the needle tip oriented tangentially to the corneal surface, being careful to avoid the damage of the iris.
-
Inject the MCE solution and leave the needle in place for 1 min in order to allow MCE settle in the anterior chamber and in the iridocorneal angle. Subsequently, remove the needle slowly to avoid mechanical stress of ocular tissues.
-
Instill antibiotic ointment to prevent infection and 0.5% proparacaine hydrochloride for the post-surgical analgesia.
-
Allow animal to recover anesthesia until they regain movement and monitor animal behavior within the first 24 h post-injection for possible signs of pain or distress.
Controls
The suitable control for the MCE model generally consists in the intracameral delivery of saline following the injection procedures previously described. Depending on the experimental plan, the control could be realized both in the contralateral eye of that injected with MCE (internal control) or in a separated animal (external control). However, slightly different information could be retrieved from each of the control variants. In effect, the adoption of the internal control allows to finely discern differences due to the MCE-injection within the same systemic condition but with the possibility of some interocular influence the MCE treatment on the control eye. This possibility can be excluded if adopting an external control, although possible individual differences between animals could represent a possible limitation in this case. Therefore, the realization of a dual control, both internal and external, would be desirable.
Experimental models of inherited glaucoma
The study of the glaucoma onset and progression has revealed a significant contribution of genetic factors in the context of its multifactorial pathophysiology. In effect, the glaucoma-related phenotype has been associated to genetic alterations in several genes (12). Besides the importance of clarifying the glaucoma genetic background, the individuation of the glaucoma-related genes has paved the way for the design of genetic animal models intensively used for the study of basic pathophysiological mechanisms and pharmacological interventions. These animal models allow a spontaneous IOP elevation without surgical interventions, thus avoiding manipulation-derived side effects and/or analytical biases. Considering the spontaneous and non-drastic ocular hypertension, these models are commonly suited for the analysis of the IOP modulation, resulting particularly useful to test the efficacy of hypotensive and/or neuroprotective drugs (13). They allow a high-efficiency reproduction of the glaucomatous phenotype with an optimal reproducibility. Although several large and small animals are characterized by a spontaneous onset of glaucoma, murine models are commonly used for basic and translational research. In effect, they display anatomical and functional similarities compared to human eyes and high degree of genetic conservation as compared to the human genomes, allowing the study of genetic features, risk factors, and physiological mechanisms characterizing human glaucoma with contained manipulation, breeding and housing limitations (14). Several inherited murine models mimicking different glaucoma subgroups are currently available. For instance, mice displaying a mutation in the serine protease Prss56 have been shown to develop reduction of the ocular axial length, a relatively large lens, and a shallow angle which predispose them to angle closure and high IOP mimicking the angle closure subtype of glaucoma (15). In addition, optineurin E50Ktg mice are used as an inherited mouse model of normotensive glaucoma since they do not develop elevated IOP but display the loss of RGCs of about 25% by 16 months of age (16). Although a considerable number of inherited model have been described over the past decades, myocilin mutant mice and the DBA/2J mouse strain are currently the most common murine models of inherited glaucoma (17). Myocilin mutant mice display a Tyr423His myocilin point mutation corresponding to the human MYOC Tyr437His mutation, producing a moderate IOP elevation (about 2 mmHg) at 18 months of age, which in turn drives the RGCs loss in the peripheral retina and axonal degeneration (18). This model has been extensively used for the study of the myocilin role in the onset of IOP elevation clarifying the potential role of the genetic background for the onset of the disease (19). However, the mild increase in IOP occurring in very old mice make this model less appropriated for the study of the age-dependent morpho functional alterations related to glaucomatous progression. DBA/2J is an inbred mouse strain firstly recognized in 1998 (20). It is characterized by a progressive increase in IOP due to iris abnormalities related to two recessive mutations in the glycosylated protein nmb (Gpnmb) and tyrosinase-related protein 1 (Tyrp1) genes (21). This model displays an age-dependent progression of the glaucomatous morpho functional features, thus resembling glaucoma progression in humans (6). The dynamicity of the glaucoma progression in these mice make this model optimal for retrieving outcomes with highly translational potential.
DBA/2J mouse model of glaucoma
DBA/2J mice develop elevated IOP and glaucoma with age (20). The IOP elevation and glaucoma in DBA/2J mice is natural, age-related, variable, progressive, and asynchronous (6). The IOP elevation is subsequent to an iris disease that deposits pigment in the ocular drainage structures. In particular, the recessive mutations in Gpnmb and Tyrp1 genes induce melanogenesis and a subsequent inflammatory response which drive iris cell death (22). The structural melanosome abnormalities characterizing DBA/2J mice depict a phenotype similar to that of human pigmentary glaucoma (23). However, several phenotypical hallmarks in DBA/2J retinas can be extended to other human glaucoma subgroups such as optic nerve head excavation, progressive RGCs loss, optic nerve cupping, nerve fiber layers thinning and others. Thus, the DBA/2J mouse provides a powerful model system for determining mechanisms that insult RGCs in glaucoma and for characterizing specific damaging pathways (6).
Procedure
DBA/2J mice breeding
-
Breed DBA/2J mice following the procedure provided by the Animal Care Committee of your institution.
-
Since the model is based on mice age, be careful in organizing the animal colony by age. Consider that females are more prone to develop glaucomatous phenotypic features than males (56% of females and 15% of males at 4 months of age).
-
If possible, catalog each mouse individually by assign serial numbers by mean of numbered ear tags.
Controls
The identification of an appropriated relative control for DBA/2J mice is not immediate. The use of the common reference mouse strain C57BL6/J as control is scarcely reliable when considering the genetic discrepancy which could translate into differences in neuronal activity, post- and pre-synaptic protein expression, and synaptic transmission and plasticity (24). Therefore, the use of C57BL6/J mice as control is limited to the evaluation of gross differences in structural features. Formerly, the DBA/2J itself is an inbred mouse strain and it is widely used as a control in a variety of other non-ophthalmic research fields. Considering that the pathological phenotype of DBA/2J strain derives from two alleles namely GpnmbR150X and Tyrp1isa (23), the actual strain considered as genetically matched control for DBA/2J is the DBA/2J-Gpnmb+/SjJ. This coisogenic strain has a functional allele of Gpnmb that avoids the development of the age-related IOP elevation, thus resulting an appropriate control for the assessment of pathogenic events linked with IOP elevation (25). However, the reliability of DBA/2J-Gpnmb+/SjJ as a control is significantly lower when considering exclusively the assessment of RGC functional modulation. In effect, although obvious differences in maximal pattern electroretinogram (PERG) responses could not be found between still non-glaucomatous young DBA/2J and DBA/2J-Gpnmb+/SjJ mice, some notable differences have been found in the inner retinal neural processing for spatial contrast functions, which can have a counterpart in the RGC susceptibility to insults or diseases (26). In this case, a longitudinal approach should be adopted by considering the young DBA/2J (<2 months of age) as the most appropriate control.
Analytical procedure
IOP levels
IOP is generally assessed using non-invasive methods as the rebound tonometry. This technique allows to measure the IOP in awaken or anesthetized animal without surgical interventions and subsequent animal distress. Therefore, IOP can be longitudinally monitored in each animal following experimental rationales similar to those of clinical follow-up. The reliability of the IOP assessment using the rebound tonometry is particularly dependent on few aspects: the probe orientation, the sampling procedures, and the sampling time. The probe rebound is strictly dependent on the surface stiffness, which could vary depending on the external ocular region (27). Since the instrument is usually calibrated for the corneal stiffness, the probe should always be oriented at the center of the pupil distancing about 2 mm from the corneal surface. The IOP readings could results particularly variable since they are influenced by animal breathing and movements or minimal changes in probe orientations. Particular care in IOP analysis should be made when performing tonometric recordings in animals under anesthesia since the latter could influence the actual measurements depending on both the nature of the anesthetic drug and the time after the induction (28). Therefore, multiple sampling procedures (5-10) at a fixed time after the anesthetic administration are usually preferred to ensure a reliable measure of IOP. Alternatively, IOP can also be assessed on conscious animals to exclude the influence of anesthesia but, in this case, a preliminary habituation procedure should be performed in order to reduce the IOP variations due to animals’ manipulation acute distress. IOP is also subjected to diurnal variation over the course of the day (29). Thus, the IOP measurement should be always performed at the same rage of time during the day. Given the high variability and possible limitations of the tonometry, several additional parameters strictly associated with the IOP elevation could be considered as complementary parameters aiming at further corroborating the analytical observation. For instance, imaging techniques such as whole-eye optical coherence tomography (30) and dynamic, contrast-enhanced magnetic resonance imaging (MRI) (31) could highlight alterations in eyeball morphology due to impaired aqueous humor outflow. The use of these techniques could further confirm the reliability of the observation obtained by tonometry and could function as a direct IOP-related parameter to consider when the tonometry becomes poorly reliable as the case of very advanced ages in DBA/2J (see below) (32).
RGCs activity
The assessment of retinal neuronal activity can be retrieved using electroretinographic recordings. Electroretinogram (ERG) ensure a sensitive and non-invasive analysis allowing individual longitudinal follow-up of neuronal functional status in the retina under physiological and pathological conditions (33). The electrical signal representing the retinal activity is generated by delivering visual stimuli, which may differ in terms of nature (flashes or patterns) and conditions (dark adaptation or light adaptation) and recorded by mean of a recording electrode positioned on the corneal surface and a reference electrode positioned in the animal ipsilateral scalp or cheek. The RGCs function can be retrieved by means of two specific ERG variants emphasizing the activity of inner retina neurons. Under photopic conditions (light adaptation), the ERG response displays one specific component namely photopic negative response (PhNR; belated negative wave) which has been established to correlate with the RGCs functional status in human and animal glaucoma (34). Notably, the PhNR analysis is directly influenced by the overall photopic ERG response, thus resulting less sensitive for minimal changes in RGCs activity. A more specific analysis of the RGCs activity can be obtained by the PERG (35). It consists in delivering pattern-reversal stimuli at constant mean luminance, which drive the concomitant response of RGCs receptive field organized in antagonistic regions, whereas the activity of photoreceptor organized in adjacent elements is in opposition of phase and results cancelled out at the electrode. The PERG assessment allows the analysis of minimal changes in RGCs activity, also preceding an overt cellular degeneration (36). PERG is generally analyzed considering both its positive (N35-P50) and negative (P50-N95) components.
RGCs density
The heterogeneity among RGCs subgroups and the similarities with other retinal neurons such as amacrine cells, make the morphological and densitometrical analysis of this cell type not immediate. In the last years the immunohistochemical approach has been established as the most appropriate for the preservation of RGCs integrity and for its analytical reliability (37). However, several RGCs-specific markers with possible different outcomes can be considered for the actual analysis of RGCs density. NeuN, Thy1 and substance P are among the protein consider as specific marker for RGCs (38). However, their immunostaining often is limited to a subgroup of RGCs, and they can also include other retinal cells, such as displaced amacrine cells (37). More reliable outcomes can be obtained from the immunostaining of transcription factors, such as Brn3a, as well as RNA-binding proteins that are specific to RGCs such as RNA-binding protein with multiple splicing (RBPMS) (39,40). Both have been established as excellent marker for the densitometric analysis of RGCs in the retina. Brn3a has been found to be expressed in about 90% of RGCs in mouse and rat retinas. However, 20% more RBPMS-immunoreactive somata then Brn3a somata have been found in mouse and rat retinas (37).
Outcomes
MCE model of induced IOP elevation
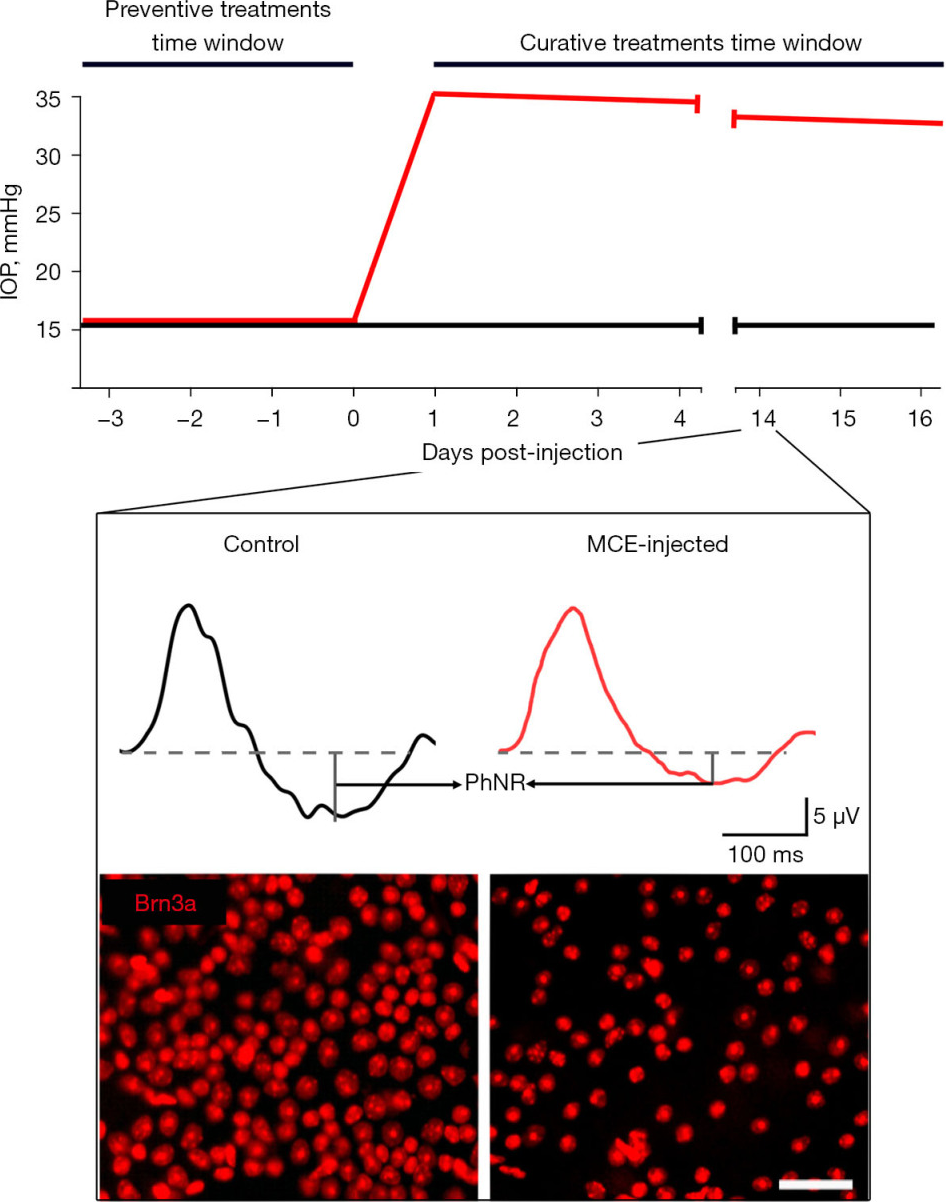
Typical outcomes of the MCE model are reported in Figure 1. A significant increase in IOP is perceivable in MCE-injected mice within the following 24 h. The MCE injection allows to reach IOP levels about 2.0-fold higher than those prior the surgical intervention. Although the hypertensive condition is maintained, the IOP classically undergoes a slight decrease over-time of about 5% per-week possibly given by a gradual loss of MCE hypertensive effect. The injection of MCE does not alter the activity of the outer retina neurons since both the photopic a-wave (early negative component referring to photoreceptors activity) and b-wave (positive component related with mid retina activity) are classically altered. Conversely, the MCE injection determines a drastic drop in PhNR amplitude, which could reach the 50% of the baseline values at 2 weeks after the injection. The loss of RGCs activity at 2 weeks following the MCE injection is also reflected by the PERG response which results halved. The MCE injection has been found to drastically affect the RGCs density. In effect, mice injected with MCE display a significant loss of Brn3a-positive cells resulting decreased of about 40% after 2 weeks from the onset of ocular hypertension (41). The reliability of the present model in displaying glaucomatous manifestations mainly depends on the efficacy of the surgical procedure itself. In effect, the operator should have particular care in let MCE settle on the anterior chamber since possible outpours following the injection could results in a lower amount of viscous fluid entering the anterior chamber and subsequent less effective alteration in the aqueous humor outflow. Generally, the efficiency of IOP elevation over 15 mmHg following MCE injection is high, while the IOP measured at the peak and the subsequent IOP-dependent retinal alterations may vary depending on the actual amount of MCE that has effectively settled in the anterior chamber. For this reason, the outcomes related to the MCE model should desirably be analyzed by correlating the individual IOP with the deriving degenerative phenomena.
Figure 1 Summary of the typical outcomes deriving from the MCE-induced model of IOP elevation. Graphical representation of the time-dependent profile of IOP in control (black line) and following the intracameral injection of MCE (red line). The inset shows typical differences at 14 days post-injection between control and MCE-treated mice in PhNR retrieved from photopic ERG traces and in the density of RGCs immunolabeled for Brn3a. Scale bar: 50 μm. IOP, intraocular pressure; MCE, methyl cellulose; PhNR, photopic negative response; ERG, electroretinogram; RGCs, retinal ganglion cells.
DBA/2J mouse model of glaucoma
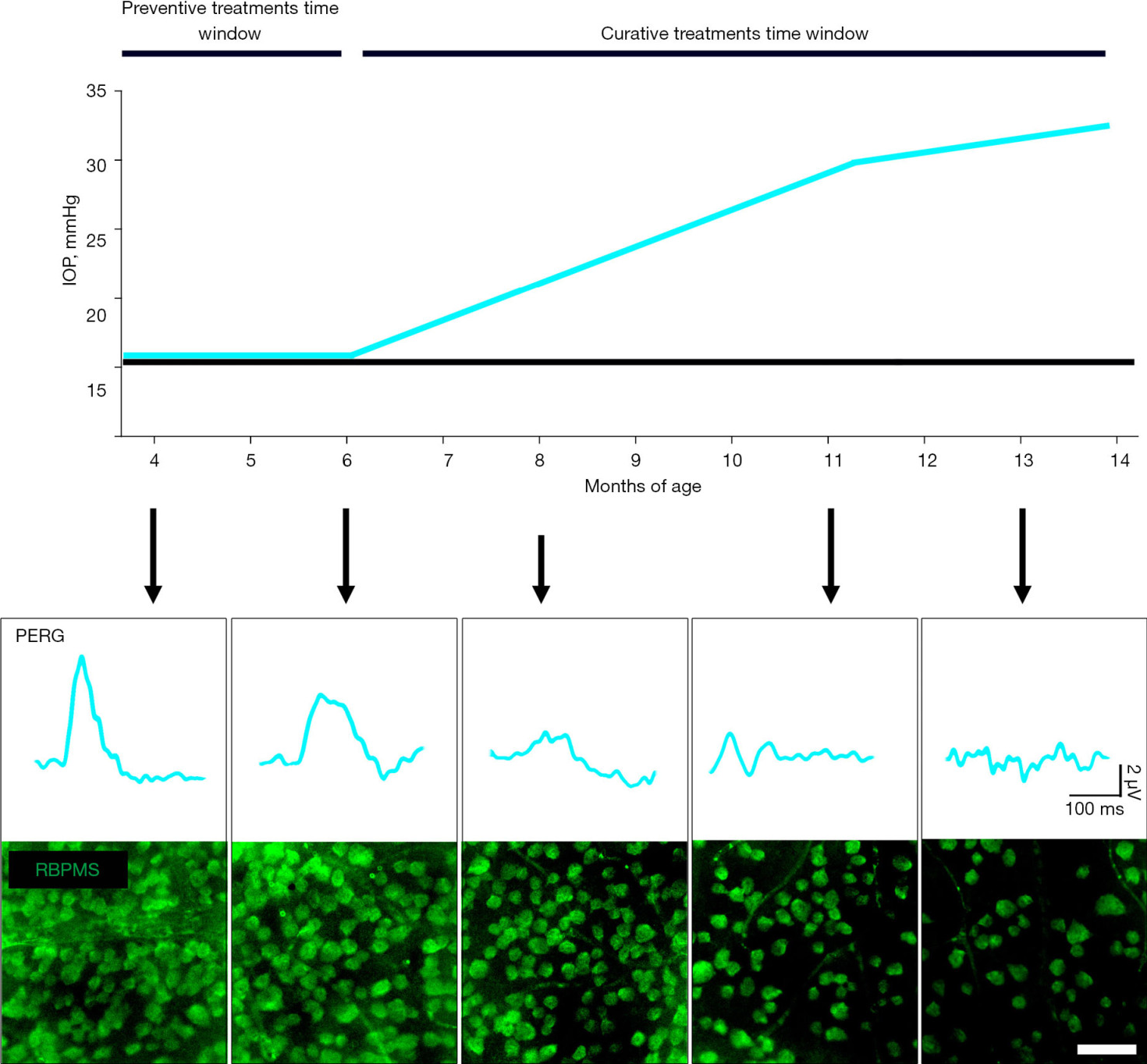
DBA/2J mice are characterized by iris abnormalities that have been found strictly correlated with the onset of ocular hypertension. In effect, the dispersion of iris pigment and cell debris in the anterior chamber causes the impairment of aqueous drainage, thus increasing the anterior chamber hydrostatic pressure (22). The time-dependent progression of IOP increment and relative RGCs alterations are summarized in Figure 2. The increase in IOP is perceivable starting at 6 months of age and displays a continuous age-dependent increase of 2–3 mmHg per month (42) The large amount of evidence regarding RGCs activity in DBA/2J derive from PERG analysis. A strong correlation between PERG dysfunction and increase in IOP is detectable in this model. In particular, it has been described that IOP higher than 30 mmHg reached around 11 months of age in DBA/2J corresponds to PERG amplitudes close to the noise level (43). Interestingly, longitudinal PERG analysis in this model have revealed that PERG alterations precede the overt increase in IOP starting at 4 months of age, suggesting possible degenerative mechanisms in this model occurring independently from the ocular hypertension (4). This feature of the DBA/2J mouse model is currently under investigation in order to clarify whether this model could also be suitable for the study of IOP-independent mechanisms occurring in early stages of the disease or in normotensive glaucoma. Structural alterations typical of glaucoma phenotype become evident after 8 months of age in DBA/2J mice. In effect, the RBPMS-positive RGCs density in DBA/2J mice retinas results significantly reduced of about 50% at 8 months of age and 70% at 13 months of age (44,45). This correlates with other structural alterations such as nerve fiber layer thinning and loss of axons count both resulting halved at 11 months of age in DBA/2J mice (44). Subsequently, the axonal transport result decreased of over 95% at 14 months of age (46).
Figure 2 Summary of the typical outcomes deriving from the DBA/2J inherited mouse model of glaucoma. Graphical representation of the age-dependent profile of IOP in normotensive mice (black line) and DBA/2J mice. Arrows indicate the age-matched outcomes of RGCs activity assessed with PERG and density of RGCs immunolabeled for RBPMS. Scale bar: 50 μm. IOP, intraocular pressure; PERG, pattern electroretinogram; RBPMS, RNA-binding protein with multiple splicing; RGCs, retinal ganglion cells.
The development of glaucomatous phenotype in DBA/2J mice is highly variable in onset and entity. In effect, the occurrence of IOP elevation may occur later than 6 months of age or even not occur in some cases. Moreover, the IOP reached during the hypertensive stage, although higher than 15 mmHg, may be extremely variable, thus influencing the variability of the downstream IOP-dependent phenomena (47). Almost all the glaucomatous features on the DBA/2J mouse model displays an age-dependent progression. Therefore, side phenomena typical of aging might limit the interpretation of functional and/or structural outcomes. For instance, pupil dilation deficits, corneal opacification, or corneal calcification typical of aged DBA/2J could hinder the reliability of IOP and PERG analysis. Additionally, belated alterations in the light detection and synaptic transmission have been suggested by evidence of belated outer retinal reorganization (48). Noteworthy, these are typical features of the very advanced ages (>12 months) that should be taken into account when analyzing the glaucomatous progression in the DBA/2J model.
Conclusions
The study of glaucoma pathophysiology and its experimental modeling are mutually influenced and still in continuous development. The availability of a single gold standard model for the experimental reproduction of glaucoma is far to be reached and, possibly, cannot be obtained considering the disease extreme complexity. Therefore, the availability of several experimental model focusing and reproducing different disease features are currently needful for the integration of the deriving outcomes to reconstruct the actual disease pathophysiology. In this manuscript, I reported the description of procedures, analysis and typical outcomes of the MCE-induced IOP elevation and the DBA/2J mouse strain as two models, whose the deriving information about the IOP-driven neurodegenerative mechanisms rather than IOP-independent neurodegeneration and physiological mechanism leading to the IOP elevation could be coupled for the basic and translational study of glaucoma.
Acknowledgments
I am grateful to Dr. Alessio Canovai and Dr. Alberto Melecchi for their crucial suggestions and critical reading of the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dario Rusciano) for the series “Preclinical Models in Ophthalmic Research” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-21-48/coif). The series “Preclinical Models in Ophthalmic Research” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.